Type 2 diabetes research heavily relies on the BKS-db mice, and blood glucose testing is a fundamental part of this research. As test results are of the utmost importance to the success of your project, special consideration should be given to the techniques with which you use to test.
This can include controlling environmental factors, mouse conditions, timing of measurements, fasting duration, and more, which are all essential to ensure the accuracy and reliability of experimental results.
This article will outline the essential steps and considerations for conducting blood glucose tests in BKS-db mice for a few different use cases, providing researchers with valuable insights into utilizing this experimental model effectively.
*Please note that the experimental methods and procedures described in this article are based on general principles and consensus, and may require adjustments based on specific research objectives, experimental conditions, and animal models.
Postprandial Blood Glucose Testing:
- Remove the breeding cage box from the rack and place it gently on the table. Open the cage box, remove the water bottle, and transfer all the mice to be tested from the cage box to the grid with gentle and slow movements to minimize stress to the mice.
- Insert the blood glucose test strip into the glucose meter for later use.
- With the left hand, grasp the tail tip of a mouse and hold a 1 mL blood collection needle with the right hand. Align the needle with the tail tip about 1-2 mm away, and insert the needle along the long axis of the tail for about 1-2 mm.
- Release the 1 mL blood collection needle with the right hand, use the thumb and forefinger to gently squeeze the base of the tail towards the tail tip to express blood, gently squeezing out the first drop of blood (avoid squeezing the tail too hard). Immediately drop the second drop of blood squeezed from the tail tip onto the blood glucose test strip and wait for the glucose meter to display the result.
- Once the cage-wide testing is finished, refill the feed, replenish the water bottle, cover the cage, return it to the rack, and move on to the next cage for testing.
Fasting Blood Glucose Testing:
- Initiate fasting in the morning (typically before 9 AM) for a period of 5-6 hours. Follow the fasting protocol: Transfer the mice from their initial cage to a fresh one (i.e., change the bedding to eliminate any remaining feed remnants, as the original bedding may still contain them, and the mice could ingest it, affecting blood glucose test results). Discard the original feed, replace it with a new one, reattach the water bottle, cover the cage, return it to the rack, and document the fasting duration.
- Performing blood glucose testing follows the previously outlined procedure.
The blood glucose testing procedure itself is not complicated; the key lies in the details of animal husbandry and experimental procedures. Paying careful attention to these details is crucial to ensuring the accuracy of experimental results.
Note:
1. Experimental Procedures:
- Prior to blood glucose testing, maintaining a tranquil environment is imperative. Avoid any disturbance, including cage changes, to prevent potential fluctuations in blood glucose levels due to mouse stress and activity.
- Throughout the testing procedure, emphasize gentle movements. Refrain from forcefully restraining mice by tail grabbing to minimize stress and allow the mice to move freely on the grid.
- As the circadian rhythm of mice is opposite to that of humans, postprandial blood glucose testing is generally completed within 2 hours after the facility lights are turned on in the morning of the same day.
- When collecting blood from the mouse tail, preferentially measure the second blood drop. The first drop may contain tissue fluid, influencing blood glucose measurements and yielding slightly lower readings than the actual levels.
- Consistency in the testing time intervals for different sampling points within the same experiment is essential, along with the utilization of the same blood collection method. If data comparison between different experiments is necessary, it is also crucial to ensure uniform testing time frame across experiments.
- If an abnormal result occurs due to factors like low blood volume, equipment issues, or mouse stress, conduct an immediate re-squeezing and retest. If the result persists as abnormal, place the mouse back into its original cage, wait for approximately ten minutes, and then retest.
- If blood glucose testing surpasses the upper limit of the glucose meter, record it as the upper limit value (e.g., 33.3 mmol/L for human glucose meters). Alternatively, consider collecting a small blood volume (approximately 30-40 μL whole blood), extract plasma, and dilute it for testing using a blood biochemistry analyzer.
- Due to accelerated metabolic rates in mice, prolonged fasting, especially overnight, can lead to significant weight reduction (approximately 15%). Exercise caution when employing overnight fasting if continuous weight assessment is part of the experimental objective. Prolonged fasting depletes glycogen in mouse tissues, making it suitable for studying tissue glucose uptake capacity. Therefore, a short-term morning fasting of 5-6 hours provides a more physiologically relevant assessment of insulin-related effects.
- Recommended Testing Frequency: For random blood glucose testing using tail-tip needle pricking, conducting 1-5 tests per week is generally advisable. For fasting blood glucose testing using tail-tip needle pricking, one test per week is typically recommended.
2. Animal Husbandry:
Given the “three P” symptoms observed in diabetic mice, (polydipsia, polyphagia, polyuria, and weight loss), special attention is required in their daily care.
- It is recommended to house diabetic mice in SPF-grade animal facilities to prevent the presence of pathogens, which may potentially impede or delay the onset of diabetes.
- Ensure an ample supply of food and water, conducting regular checks to maintain sufficient provisions (considering the difficulties obese mice may encounter in locating food and water).
- Due to the polyuria characteristic, mouse cages may become damp and soiled, impacting mouse health during maintenance. It is advisable to increase the frequency of bedding changes, typically changing it every 1-2 days. When handling, minimize stress to mice to reduce stress responses.
- Avoid initiating experiments immediately upon the arrival of mice; adaptation to the environment is crucial, requiring at least a one-week acclimation period.
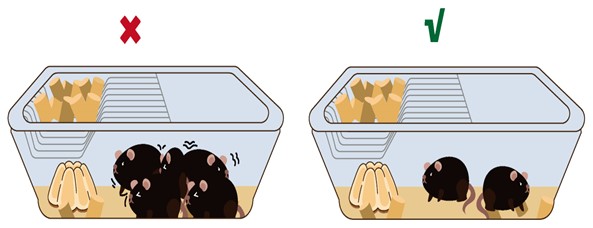
- Adjust housing density appropriately, generally accommodating 2-3 mice per cage.
GemPharmatech is proud to introduce the upgraded T056480 BKS-db v2. In comparison to its predecessor, the second generation exhibits a higher incidence of Type 2 diabetes with enhanced data stability. Learn more about T056480 BKS-db v2.

